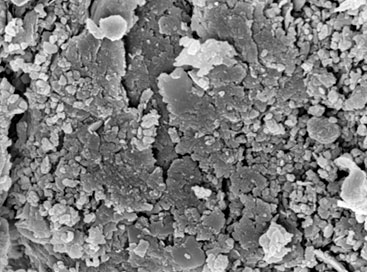
Red mud particle adsorbent and its adsorption mechanism for phosphate ion
DOI:
https://doi.org/10.35624/jminer2019.01.12Resumen
There is a large amount of phosphorus in the flotation tailwater of phosphate (PW), which will cause eutrophication of water body if discharged directly, effective removal of phosphorus is of great importance in prevention of eutrophication. In this article, we report a Non-thermally activated red mud particle adsorbent (ARMPA) for removal of phosphate, the main mineral components were hematite, calcite, oolitic chlorite, katenite and calcium nepheline and the adsorbent was found to have a pH 9.25, a specific surface area of 40.54 m2/g, a pore volume of 2.10 cm3/g, a compressive strength of 1.31 KPa, and an immersion pulverization rate of 3.72% at 24 h. This adsorbent can be used to adsorb phosphate ions (P) in PW. When the initial total phosphorus (TP) concentration was 156.7 mg/L, the amount of ARMPA was 4 g/L, and the adsorption time was 10 h, the adsorption capacity and removal efficiency of the TP were 38.46 mg/g and 98.17%, respectively. The zeta potential, XRF, SEM, FT-IR,and XPS analysis showed that when pH 8~9, P mainly existed in the form of HPO42- and PO43-. These ions reacted with Ca2+/Na+/Al3+/Mg2+, etc., and formed strong chemical bonds through surface deposition and ion exchange, which then distributed on the inner surface of the ARMPA channels. The adsorption of TP by ARMPA accords with the pseudo-first-order dynamics model, and the Langmuir model can better describe the adsorption process. Phosphorus, fluorine and other toxicity indicators in the tail water after adsorption have reached the standard requirements. After adsorption of phosphate material, it is equivalent to using phosphoric acid tailwater to comprehensively modify alkaline red mud, so as to achieve the purpose of treating waste with waste
Descargas
Publicado
Número
Sección
Licencia
<a rel="license" href="http://creativecommons.org/licenses/by-nc-nd/4.0/"><img alt="Licencia Creative Commons" style="border-width:0" src="https://i.creativecommons.org/l/by-nc-nd/4.0/88x31.png" /></a><br />Esta obra está bajo una <a rel="license" href="http://creativecommons.org/licenses/by-nc-nd/4.0/">Licencia Creative Commons Atribución-NoComercial-SinDerivadas 4.0 Internacional</a>.